HACCP
A specially designed HACCP system for users whose goal is to take maximum care of food handling conditions, meet legal standards, automatically control the process and clearly divide responsibility. This system significantly contributes to food safety and the effectiveness of HACCP procedures, which is extremely important for all participants in the food supply chain.
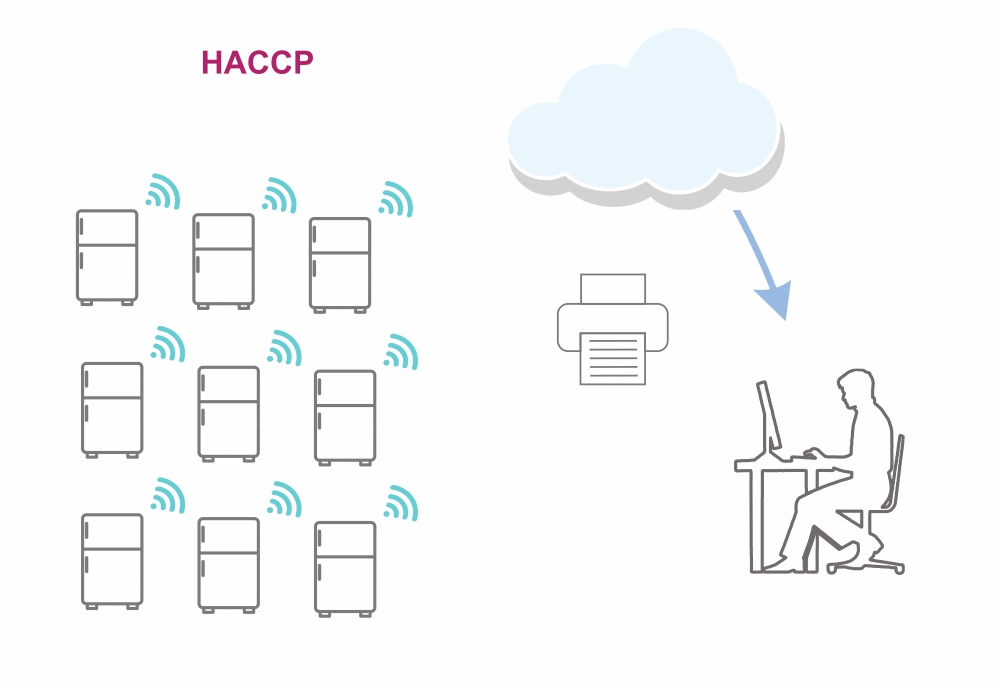
Working principle: Sensors positioned around the refrigerators continuously measure the temperature and send the data via WiFi to the web application.
Data processing: The web application stores and processes received data, monitors temperature changes and reports, and keeps a detailed record of readings on forms.
Status notification: The system sends notifications about temperatures by position in real time, to precisely specified persons in charge of a specific location, for a specific time.
Confirmation of readings: Responsible persons receive information electronically and authorize readings with one click and thereby confirm their own information.
Recording of read values: The application automatically records confirmation or non-authorization of readings.
Escalation of the problem: In the event that the responsible person does not confirm the reading within the given period, the system automatically informs the authorities about the omission, so that the necessary measures can be taken.
Automating data tracking and recording reduces human error and enables faster response to potential problems.
Detailed records of all readings and reactions enable easy monitoring and auditing of HACCP procedures.
Real-time notifications and problem escalation allow for rapid intervention, reducing the risk of food spoilage and other hazards.
Automated record- keeping helps meet legal requirements and food safety standards.
HACCP Process Tracking
LOT Number - A LOT number is a unique identifier assigned to a defined quantity of product or raw material manufactured or packed under the same conditions.
The LOT number is the cornerstone of traceability in HACCP. It links each unit of food to the exact production or packaging conditions under which it was created. Manufacturers often design LOT numbers using combinations of production dates, shifts, equipment codes, or sequential identifiers. This allows for rapid product recall in case of non-conformity or safety issues. Every incoming raw material must arrive with a LOT number from the supplier, and every outgoing finished product must be labeled with its LOT. HACCP documentation such as receiving logs, storage records, and distribution notes must all reference LOT numbers consistently. In computerized systems, the LOT is connected to storage location, shelf-life, and certificates of analysis. A clear LOT system ensures transparency, accountability, and the ability to isolate specific product groups within hours if necessary. Example: LOT 250827-B2 could mean “27 August 2025, Shift B, Line 2.”
Batch / Production Lot - A batch is the entire quantity of product produced in a single uninterrupted process under the same operating conditions.
A batch represents a complete production run that shares identical parameters such as raw materials, equipment, and time of manufacture. Each batch is normally assigned a LOT number, and in some cases multiple LOT numbers if it is subdivided. Batches are important because if a safety hazard or defect is discovered, the entire batch is considered affected and subject to quarantine or recall. Records for each batch must include the raw material LOT numbers used, critical processing parameters (e.g., cooking time and temperature), and results of quality checks. In industries such as dairy or bakery, a batch might last only a few hours, while in large-scale beverage or grain processing it can represent thousands of liters or tons. HACCP teams analyze risks by batch because it is the logical unit for containment. Without clear batch identification, traceability collapses and corrective actions become ineffective.
Production Date - / Packaging Date / Expiry Date Dates on a product show when it was manufactured, packed, and until when it remains safe or of acceptable quality.
The production date indicates when the product was manufactured. The packaging date indicates when it was packed for distribution. Expiry dates are shown as either “Use by” (for safety – applied to perishable, microbiologically sensitive foods) or “Best before” (for quality – the product may still be safe afterward but not at peak quality). These dates are vital for consumer safety and inventory management. HACCP relies on these dates to ensure FIFO (First In, First Out) or FEFO (First Expired, First Out) systems are respected. If products surpass their expiry dates, they are legally considered unsafe and must be removed from circulation. Dates must be clearly printed, legible, and accurate. Incorrect or missing date coding is a major non-conformity. Example: “Produced 25/08/2025, Use by 02/09/2025” on fresh meat. Proper date labeling supports traceability and prevents unsafe products from reaching the consumer.
FIFO and FEFO - FIFO means “First In, First Out,” while FEFO means “First Expired, First Out”; both are inventory management principles to control product flow.
FIFO ensures that older stock is always used before newer arrivals. This principle is especially important in dry goods warehouses where shelf life is long. FEFO, on the other hand, gives priority to items with the earliest expiry date, regardless of arrival date. FEFO is critical for chilled or frozen raw materials and ready-to-eat foods where shelf life is short. In HACCP, both FIFO and FEFO help prevent expired or unsafe items from entering production or distribution. Warehouse staff must arrange shelves so that the correct sequence is easy to follow. Digital warehouse systems often highlight which LOT should be dispatched first. Poor implementation of FIFO/FEFO can lead to wastage, food safety risks, and regulatory non-compliance. Auditors typically verify compliance by checking warehouse stock rotation. Both systems are preventative controls against expired or spoiled food.
Product Code - / SKU / EAN / PLU Product codes are identifiers used internally or externally to track items in warehouses, production, and retail.
SKU (Stock Keeping Unit) is an internal code companies use to manage stock items. EAN or UPC barcodes are internationally standardized numbers printed on packaging to allow for automated scanning. PLU (Price Look-Up) codes are mainly used in retail for unpackaged items such as fruits and vegetables. Within HACCP, product codes connect finished goods to their LOT numbers, recipes, and documentation. When a complaint or recall arises, the SKU or EAN leads directly to the relevant LOT data. Internal coding systems also help avoid mix-ups in warehouses with thousands of items. For consumer distribution, barcodes are mandatory on packaged goods, while PLUs help retail workers identify fresh items quickly and consistently. If products lack proper coding, traceability breaks down. Example: SKU-01455 = “Whole Milk 1L,” EAN = scannable barcode on the carton.
Raw Material - Code and Supplier Code Internal identifiers for raw materials and their suppliers, used for purchasing, inventory, and traceability.
Every raw material should be given a unique internal code in the HACCP system, and each supplier should also have a code. This makes it easy to connect incoming raw materials to the supplier who provided them. In case of a safety issue or non-conformity, the supplier code allows the manufacturer to identify the exact source. These codes are used in receiving logs, warehouse systems, and production records. Large companies typically maintain catalogs of raw materials and supplier codes for consistency. This prevents confusion when different suppliers provide similar products. During audits, inspectors often request proof that raw material LOT numbers can be linked to a specific supplier through coding. Without this system, traceability and accountability are weakened. Example: raw material “Refined Sugar” = code RM-025; supplier “SweetCo Ltd.” = SUP-018.
Purchase Order - (PO) / Delivery Note / Goods Receipt These are documentation identifiers that accompany raw materials from supplier to warehouse.
A Purchase Order (PO) is issued by the buyer to the supplier and is the first link in the documentation chain. The Delivery Note accompanies goods during transport, and the Goods Receipt confirms acceptance at the warehouse. HACCP relies on these documents because they connect raw materials with dates, LOT numbers, and suppliers. If a defect is found in a raw material, the PO and delivery note allow the company to trace the exact shipment. These documents may also include expiry dates, storage instructions, and certificates of analysis. In computerized systems, PO numbers are automatically generated and linked to inventory records. They serve as critical checkpoints in the traceability chain. Without proper documentation, raw materials cannot be reliably traced back to their origin. Example: PO-2025-118 = the 118th purchase order issued in 2025.
Warehouse Location - (Aisle/Row/Shelf/Bin) Exact address codes are used to record where each raw material or product is stored. Every item in a HACCP-controlled warehouse must have a clearly recorded storage location.
This is usually expressed as a code combining aisle, row, shelf, and bin. This system allows for quick retrieval of goods, effective rotation under FIFO/FEFO, and efficient traceability. If a product must be quarantined, the warehouse location allows staff to immediately identify where it is. Location coding is essential when warehouses hold thousands of pallets or containers. HACCP requires that incompatible items (e.g., allergens, chemicals, finished goods) be separated into designated zones, often defined within the location coding system. Digital Warehouse Management Systems (WMS) track LOT numbers and their exact storage positions. Example: HLA-R02-S03-B11 = Cold Storage A, Row 2, Shelf 3, Bin 11. This prevents mix-ups and ensures safe, organized storage.
Storage Conditions - (Temperature, Humidity, Zone) Conditions such as temperature, relative humidity, and segregation zones are controlled and recorded to maintain food safety.
Storage conditions are a critical aspect of HACCP because they directly affect product safety and shelf life. Temperature must be continuously monitored: frozen goods at –18 °C or below, chilled goods at 0–4 °C, dry goods at ≤25 °C. Relative humidity is also monitored, especially for dry products that may clump or mold if exposed to excessive moisture. HACCP requires physical separation of incompatible goods, such as allergens, cleaning chemicals, and ready-to-eat products. Storage zones are color-coded or clearly marked. Staff must record temperature and humidity readings in daily logs or automated systems. Deviations trigger corrective actions such as isolating the product, investigating the cause, and possibly discarding the affected LOT. Example: chilled raw fish stored at 2 °C with daily humidity checks to avoid condensation and bacterial growth.
Measuring Device ID - and Calibration Status Each thermometer, logger, or scale must have its own ID and calibration record. In HACCP, measuring devices are essential for verifying critical limits.
Each instrument must be uniquely identified with an ID label. Calibration records ensure that the device gives accurate results. For example, a thermometer used to verify cooking temperatures must be checked regularly against a certified reference. Calibration dates, due dates, and certificates are recorded in the HACCP file. During monitoring, staff must note the device ID alongside the measurement. This provides proof that the reading was taken with a verified instrument. Uncalibrated devices can lead to false results, putting food safety at risk. Regulatory audits often include inspection of calibration logs. Example: “T-LOGGER-014, calibrated 10/05/2025, next calibration due 10/11/2025.” Proper calibration management ensures trustworthy data and compliance.
CCP / CP / OPRP / PRP - Different categories of controls within HACCP distinguish critical points, operational measures, and prerequisite programs.
A CCP (Critical Control Point) is a stage where control is essential to prevent or eliminate a food safety hazard (e.g., cooking to destroy pathogens). CP (Control Point) is important for quality but not directly critical for safety. OPRP (Operational Prerequisite Program) is a specific preventive measure that controls hazards indirectly (e.g., sieving flour, using magnets). PRPs (Prerequisite Programs) are the general hygiene foundations such as sanitation, pest control, water quality, and staff training. Each type of control has its own monitoring and documentation requirements. Identifying CCPs is the core of HACCP hazard analysis. CCPs must have measurable critical limits, while PRPs are more general practices. Together, CCPs, OPRPs, and PRPs form the complete HACCP safety net. Misclassification of points leads to ineffective control and audit failures.
Critical Limits (CL) - Measurable values at CCPs that separate safe from unsafe conditions. Critical limits are the thresholds that must not be exceeded at critical control points.
For example, cooking chicken must achieve a core temperature of ≥75 °C for 2 minutes. If this condition is not met, the product is considered unsafe. Critical limits are based on scientific data, regulations, or industry standards. In HACCP, every CCP must have a defined CL. Staff are trained to measure and record the parameter (temperature, pH, water activity, etc.) with precision. Deviations require immediate corrective actions, such as reprocessing or discarding the product. Auditors always check if CLs are scientifically justified and consistently applied. Example: pasteurization of milk at 72 °C for 15 seconds. Without clear CLs, monitoring is meaningless and food safety is compromised.
Monitoring / Frequency / Signature - Monitoring means regularly checking parameters, noting how often, and who performed the task.
HACCP monitoring ensures that CCPs and other controls are functioning correctly.
Each monitoring record must specify the frequency (e.g., every batch, every hour, daily), the method (manual or automated), and the person responsible. Signatures or initials prove accountability. If electronic systems are used, a digital ID replaces the signature. Consistent monitoring demonstrates due diligence and compliance. Gaps or missing signatures are considered non-conformities. Monitoring must be frequent enough to detect deviations before unsafe product is released. Example: “Chill room temp: measured every 4 hours, signed by operator J.D., verified by supervisor daily.” Monitoring records also provide evidence in case of complaints or investigations.
Core Temperature / Ambient Temperature / Time–Temperature - Measurements of product core, environment, and holding times ensure safe thermal processes.
Core temperature is the internal temperature of the product, usually measured with a probe. Ambient temperature refers to the temperature of the environment, such as a cold room or oven chamber. Time–temperature combinations are used to verify heat treatments: not only must the product reach the required temperature, but it must hold it for a defined time. In HACCP, thermal processes such as cooking, pasteurization, or cooling are typically CCPs with strict critical limits. Operators must record both core and ambient temperatures as well as duration. Failure to meet these requirements means the product cannot be released. Example: poultry core temp ≥75 °C for 2 min; oven ambient 180 °C. Without precise measurement, pathogens may survive, endangering consumers.
pH, Water Activity (aW), Salt, Solutes -Physicochemical parameters that influence microbial growth and product safety.
The acidity (pH), water activity (aW), and salt or solute concentration determine whether bacteria, yeasts, or molds can grow. HACCP systems often use these values as preventive controls or CCPs for shelf-stable foods. For example, jams rely on low aW, pickles on low pH, cured meats on salt. Parameters are measured using pH meters, aW meters, or chemical analysis. Results must be recorded against each batch. If values are outside safe limits, the product cannot be released. Scientific references provide the acceptable ranges. Example: pH ≤4.6 prevents Clostridium botulinum growth. Consistent monitoring ensures stability and consumer safety. Incorrect measurements or lack of calibration can lead to dangerous products being placed on the market.
Cleaning / Disinfectant Concentration -Concentrations of cleaning and disinfection solutions must be measured, recorded, and verified.
In HACCP, cleaning and disinfection are prerequisite programs essential for food safety. Chemicals must be prepared at the correct concentration to be effective but not harmful. Operators measure concentrations with test strips, titration kits, or digital devices. Records include the LOT number of the cleaning agent, concentration achieved, contact time, and temperature of solution. Incorrect concentrations can leave pathogens alive or cause chemical contamination. Supervisors must verify logs regularly. Example: chlorine solution at 100 ppm, tested before use. Over- or under-concentration is considered a non-conformity and requires corrective action. This ensures equipment, surfaces, and utensils are hygienically safe for food contact.
Certificates: CoA / CoC / SDS -Documentation provided by suppliers to prove compliance, analysis, and safety.
A Certificate of Analysis (CoA) provides laboratory test results confirming raw material specifications (e.g., microbiology, composition). A Certificate of Conformity (CoC) states that the material complies with the agreed specification or legal standard. Safety Data Sheets (SDS) provide health and safety information for chemicals. HACCP requires that all incoming raw materials are accompanied by these documents. They are stored in the quality assurance system and linked to LOT numbers. If a supplier cannot provide them, the material may be rejected. During audits, inspectors often request to see certificates for selected LOTs. Proper documentation supports supplier approval programs and demonstrates due diligence. Example: sugar shipment arrives with CoA showing moisture content, ash, and microbiological results.
Material Status: Released / Hold / Reject / Rework -Status labels applied to raw materials or products indicating their usability.
Every batch or LOT must carry a clear status. “Released” means approved for use. “Hold” means quarantined pending investigation (e.g., awaiting test results). “Reject” means the material has failed and must not be used. “Rework” means the material can be reprocessed according to a defined procedure. HACCP requires physical and visual separation of materials under different statuses, often with colored labels or designated storage areas. Documentation must match the status label to prevent mistakes. Unauthorized use of “Hold” or “Reject” material is a critical non-conformity. Example: pallet of flour placed on “Hold” until aflatoxin test results are received. Status control ensures only safe and approved materials enter the production process.
Allergen Labeling and Segregation -Allergens must be clearly identified, labeled, and stored separately to prevent cross-contamination.
Allergens are one of the most serious risks in HACCP because even small traces can cause severe reactions. HACCP requires clear labeling of allergenic raw materials and products (e.g., milk, gluten, peanuts). Warehouses must segregate allergens into dedicated zones or shelves, often using colored stickers or containers. Production lines may need separate equipment, or strict cleaning procedures between runs. Staff must be trained to handle allergens carefully and avoid cross-contact. Allergen information must appear on product labels according to legislation. Failure to control allergens is a critical non-conformity and can lead to recalls and consumer harm. Example: flour containing gluten stored in a separate, clearly marked zone, with tools color-coded for allergen handling.
Pest Control – Trap ID and Map -Each pest control device must have an ID and be recorded on a site map with inspection results.
Pest control is a prerequisite program in HACCP. All traps, baits, or monitoring devices are numbered and shown on a facility map. Pest control records must include trap ID, date checked, findings, and corrective actions. Inspections are performed regularly by trained staff or contracted specialists. HACCP auditors often request the pest control map and logs. Any signs of pests must trigger immediate corrective measures such as cleaning, sealing entry points, or chemical treatment. Trap IDs prevent confusion and ensure that each device is consistently monitored. Example: Trap #14, checked 12/08/2025, result: no activity. Pest control is essential to prevent contamination of food by rodents, insects, or birds.
Metal Detector / Sieve / Magnet – Test Bodies -Control devices for foreign bodies must be tested regularly with standard test pieces.
Metal detectors, sieves, and magnets are common operational controls in HACCP. They must be tested at defined intervals to ensure they are functioning correctly. Test bodies (calibrated pieces of ferrous, non-ferrous, and stainless steel) are passed through the detector, and results (pass/fail) are recorded. Sieves and magnets must be checked for integrity and effectiveness. Records include test frequency, test body size, and outcome. Failure of a test means production must stop, and affected product must be isolated until the issue is resolved. HACCP auditors require evidence of regular testing. Example: metal detector tested every 2 hours with 2 mm Fe, 2.5 mm NFe, and 3 mm SS test pieces. This ensures consumer safety from physical hazards.
CIP / SIP and SSOP -Cleaning-In-Place (CIP), Sterilization-In-Place (SIP), and Sanitation Standard Operating Procedures (SSOP) describe hygiene programs.
CIP and SIP are automated cleaning and sterilization processes for tanks, pipelines, and closed systems. They run defined cycles with specific time, temperature, and chemical concentration. SSOPs are detailed written instructions for manual cleaning tasks. HACCP requires documented cleaning procedures to prevent contamination and maintain hygiene. Records must show date, time, operator, chemicals used, concentration, and verification (e.g., swab test results). Failure to follow cleaning protocols can result in microbial contamination. Example: daily SSOP for slicing machine: disassemble, clean with 100 ppm chlorine, rinse, sanitize, reassemble, sign-off by supervisor. Cleaning programs are fundamental PRPs in HACCP.
Line, Shift, and Operator Identification -Production records must show which line, shift, and operator performed each task.
Traceability requires knowing not only what was produced, but also where, when, and by whom. Each production line has an ID, each shift is labeled (A/B/C), and operators sign or log into records. This allows root cause analysis in case of problems. If an error is detected, management can identify the responsible shift or operator and investigate training or equipment issues. Recording line, shift, and operator also strengthens accountability and transparency. Example: Line L2, Shift B, Operator J.S. HACCP auditors often check for consistency between production schedules, batch records, and operator logs. Without these identifiers, corrective actions may be delayed or misdirected.
Traceability – “One Step Back, One Step Forward” -Companies must be able to trace raw materials back to suppliers and finished products forward to customers.
HACCP requires full traceability within the supply chain. “One step back” means knowing exactly which suppliers and LOT numbers entered each batch. “One step forward” means knowing which customers received products from a given LOT. Traceability tests are often conducted to ensure that companies can retrieve this information within hours. Records must connect receiving logs, production logs, and distribution records. In case of recall, this system ensures rapid, targeted withdrawal. Without it, entire markets may need to be cleared, causing huge costs. Example: flour LOT 123 from Supplier A used in bread batch 456, distributed to Stores 1–5. Efficient traceability minimizes risk and protects both company and consumer.
Complaints, Recalls, and CAPA -Systems for handling complaints, recalls, and corrective/preventive actions (CAPA) are mandatory in HACCP.
Customer complaints must be logged, investigated, and resolved. If a food safety issue is confirmed, a recall procedure is activated. Recalls must identify affected LOT numbers, customers, and distribution channels. CAPA (Corrective and Preventive Actions) are formal processes to correct the immediate issue and prevent recurrence. Records must show what happened, who was responsible, timelines, and effectiveness checks. Regulatory authorities may require evidence of recall effectiveness. HACCP audits always review complaint and recall logs. Example: Complaint received about foreign body in jam jar → investigation traces to sieve failure in Batch 567 → CAPA: replace sieve, train staff, increase test frequency. This system demonstrates responsibility and continuous improvement.
Internal Labels and Tags -Internal labels include product name, SKU, LOT, dates, allergens, storage conditions, and status.
Within HACCP, internal labeling is essential to avoid confusion and cross-contamination. Labels on pallets, containers, or intermediate products must clearly display the necessary information. This typically includes product name, SKU, LOT number, production and expiry dates, allergen information, storage instructions, net weight, line/shift, barcode or QR code, and status (Released/Hold/Reject). Labels may also include icons or colors for allergens or quarantine. If labels are unclear, illegible, or missing, errors and contamination can occur. Digital systems increasingly use QR codes linked to full electronic records. Example: Label showing Milk Powder, SKU-202, LOT 250827, Produced 27/08/2025, Use by 27/02/2026, Contains Milk, Store < 25 °C, Status: Released.
Digital Records and WMS/MES -Warehouse Management Systems (WMS) and Manufacturing Execution Systems (MES) store digital records of HACCP data.
Modern HACCP systems rely on digital tools to manage traceability. WMS manages warehouse data such as LOT numbers, storage locations, and expiry dates. MES manages production data such as batch composition, process parameters, and operator actions. Digital systems generate automatic IDs, time stamps, and user logs, which provide secure and auditable records. They reduce human error and speed up traceability tests. Many systems also integrate with temperature monitoring devices and calibration databases. Regulators and auditors increasingly expect digital traceability in large facilities. Example: MES record shows Batch 455 includes LOT 123 flour, LOT 345 yeast, produced 25/08/2025, by Operator J.S. Digital systems strengthen compliance and transparency.
Calibration and Verification Labels -Instruments must carry labels showing calibration and verification dates.
Every measuring device must have a visible label or sticker showing its calibration status. This includes thermometers, scales, pH meters, and data loggers. The label typically shows instrument ID, last calibration date, and next due date. During HACCP monitoring, staff record the instrument ID so that results can be linked to a verified device. Verification is a less formal check (e.g., daily ice-point test for a thermometer). Both calibration and verification provide assurance that readings are accurate. Auditors routinely check calibration labels and logs. Example: Scale ID 034, Calibrated 01/06/2025, Next Due 01/06/2026. Without proper calibration control, monitoring records lose reliability and may be invalid.
Color-Coded Cleaning Tools and Zones -Tools and equipment are color-coded to prevent cross-contamination between zones.
HACCP requires strict separation of hygiene zones. One effective method is to assign colors to tools for specific areas. For example, blue for raw material zones, green for finished product areas, red for allergens, and yellow for chemical storage. Staff training ensures that tools are never moved between zones. Color coding reduces risk of cross-contact and is easy to enforce visually. Auditors often check that cleaning equipment is stored correctly and matches the color coding scheme. Example: red brushes used only for allergen areas, kept in marked storage. Color coding is a practical, preventive measure supporting prerequisite hygiene programs.
Example Formats for Codes and Records -Standardized formats for LOT numbers, storage locations, CCP codes, and instrument IDs improve consistency.
Companies should establish consistent coding formats for all identifiers. LOT numbers may include date, shift, and line (e.g., LOT-L20250827-B-M3). Storage location codes may specify warehouse, row, shelf, and bin (e.g., HLA-R02-S03-B11). CCPs should be numbered and described (e.g., CCP-02 Cooking, CL ≥75 °C core). Instrument IDs should be systematic (e.g., T-LOGGER-014). Standardization prevents confusion and mistakes during data entry, audits, and recalls. Staff training must emphasize the correct use of formats. Example: A product traceability record combines SKU, LOT, and CCP codes to provide a full picture of safety control. Consistent formats support robust and efficient HACCP systems.
-